The Drop Checker by Walter Reed |
||||
|
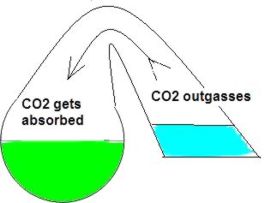
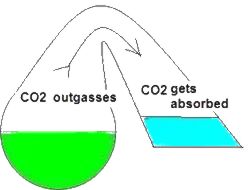
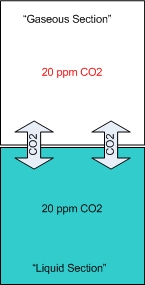
In my opinion the drop checker is one of the hottest and most misunderstood items around for the planted aquarium. This simplistic little device can give you the most accurate CO2 readings possible. However I’ve read quite a few posts on forums where this device seems to be held in some sort of mystical reverence. I hope to shed some light on this little device. First perhaps some background information to help us along. How a drop checker works A drop checker is basically a reservoir that holds an indicator solution (4 degree KH standard and a pH indicator, but more on that later), and an air space that separates the aquarium water from coming into contact with the indicator solution. When CO2 is injected, and the aquarium water contains more CO2 than the indicator solution, the injected CO2 will outgas from the aquarium water into the airspace inside the drop checker. The CO2 in the air space in the drop checker will then be absorbed into an indicator solution inside the drop checker. That absorption of CO2 into the indicator solution will then lower the pH of the KH standard, which in turn will change the color of the pH indicator. When CO2 is NOT injected into the aquarium, and the indicator solution contains more CO2 than the aquarium water, the CO2 will outgas from the indicator solution into the airspace inside the drop checker. From the airspace inside the drop checker the CO2 will get reabsorbed into the aquarium water. The out gassing of CO2 from the indicator solution inside the drop checker will raise the pH of the solution which will change the color of the pH indicator.
Why a drop checker works CO2 is a gas that goes into, and comes out of solution fairly easily and likes to reach a point of equilibrium (e.g. in a sealed bottle, the CO2 dissolved in a liquid will eventually be equal to the “air” space above the liquid). A drop checker provides an environment for CO2 to seek equilibrium and move in and out of solution inside the aquarium while providing a visual cue to its movement and concentration via the drop checker’s indicator solution.
What is pH? pH is a logarithmic measure of the acidity or alkalinity of a solution. Solutions with a pH of less than 7 are acidic, pHs greater than 7 are alkaline, and a pH of 7 is considered neutral. When we say logarithmic it means that a solution with a pH of 5 is 10 times more acidic than a solution with a pH of 6, and that a solution with a pH of 9 is 10 times more alkaline than a solution with a pH of 8. What is KH?Carbonate Hardness (KH) refers to only the bicarbonate, and carbonate anions (-charge); it does not measure the sulfates and other anions. Carbonate Hardness is a confusing term because it does not refer to hardness, but rather to the alkalinity (the ability of a solution to resist a pH change with an addition of an acid.) from the carbonates and bicarbonates. Other anions (such as hydroxide, borates, silicates, and phosphates) can contribute to the alkalinity. For the aquarium hobbyist KH is usually measured in degrees (dKH) or ppm (dKH multiplied by 17.86) What is a KH standard?For our purposes a KH standard is a solution with a known KH, and no other buffers other than carbonate and bicarbonates. A KH standard can either be purchased over the internet, or you can make your own with distilled water and baking soda. What is a pH Indicator?A pH indicator is a chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually through a color change. Probably the most common would be Bromothymol blue which happens to be the indicator used in Aquarium Pharmaceuticals standard pH test. What does this have to do with the CO2 in my aquarium?“In the beginning….” hobbyists calculated CO2 through a fixed relationship between pH, KH, and dissolved CO2. If you were to Google CO2 chart, you will be rewarded with numerous examples of the chart on the internet to show this relationship. There is also a formula that can be used: CO2 (in ppm) = 3 * KH * 10( 7-pH ) Where KH is the Carbonate Hardness measured in degrees. OK, so now what?Enter the humble drop checker. The first time I ever saw one of these was in an ADA catalog. I thought “Hey look it’s an aquarium mood ring!”. When used according to the manufacturer’s directions, which uses aquarium water, and a couple drops of reagent (a pH indicator) to make up the indicator solution, the drop checker might give you an idea of how much CO2 is in your aquarium. I say might because the indicator solutions reading will depend on what you have dissolved in your water (remember my phosphates comment above?). However, because of the separation of the aquarium water and the indicator solution you can eliminate the interference of the buffers I mentioned earlier. If you were to use a known KH reference solution made with distilled water and Sodium Bicarbonate (Baking Soda) instead of using aquarium water in the indicator solution, you can eliminate any outside buffer interference. NOTE: Now you can check the color in your drop checker against your test kit card to get a pH reading, check that against a CO2 chart and know pretty precisely how much CO2 you have in your aquarium. Coincidentally, it does not have to be a KH 4 solution, but KH of 4 does seem to have the nice transition from Blue (low CO2, high pH), Green (good CO2, pH of 6.6), yellow (too much CO2, low pH). Will a Drop Checker always be so accurate?As of the writing of this article, there are only 2 times I can think of when a drop checker will not be accurate. The first is if you using misting in your tank, andor un-dissolved CO2 bubbles get caught in your drop checker, you will get a false reading. The second (and less likely, but should be mentioned) would be if the solution became contaminated with aquarium water. This can happen with aggressive, or fast swimming fish who stray. Making a KH standard By using approximately .98 grams of baking soda (sodium bicarbonate) for each quart of distilled water, you can make your own KH standard. (Please note: this is not exact, but close enough since there are no other buffers). If your KH test is a titration test (count the number of drops till a color change), you can increase the water sample X times to increase the accuracy. e.g. I use 20 ml instead of 5 ml in my KH test and now my test kit is accurate to .25 degrees instead of a 1 degree. Drop Checker Tips
|



I’m hoping you might provide some insight on why my cal Aqua labs pearl drop checker has never changed from a blue green to dark green. I have a medium planted 50 gal. I’ve been running a pressurized co2 system for over a month. 1 drop/sec into a Red Sea co2 reactor 500. Ph is typically 7.4 and 4 deg kh. I’ve never come close to green. I’ve even tried running the co2 into my sump return creating soda water. Still very little change. My fish are not gasping at the surface. I’m wondering if my tank actually contains co2. Any suggestions?
Chris.
Per our internal discussion, you will have to provide more feedback in order to receive a valuable response.
Are you using the proper solution? Most use a 4 degree KH solution that already contains an indicator, Bromothymol Blue.
On another note, drop checkers are not very popular among NJAGC members anymore and I’m afraid that whatever feedback we can provide it will be limited when it comes to this topic. Personally I have never used a drop checker to check for CO2 levels. I always watch fish and all the other creatures to make sure they are not gasping for air or act irritated.
Thanks
Yes, I am using the provided 4 degree solution from cal labs. I did pin down my problem. Very high ph. I closely monitored my city tap water for months. Ph 7.2, GH 7 deg, KH 4 deg. I used it regularly for my water changes and top off. Come to find out the city recently changed wells. Ph 8.2, GH 10! The city worker I spoke with indicated that sometimes they miss dosing the water. I did a couple of 20% water changes with distilled water. My tank Ph is now floating around 7.2 with a GH of 4 deg. The drop checker is now responding very well. I’m sending about 1.5 drops of CO2/sec for 7 hrs with 6 hrs of light. I figure I’m at about 25 ppm Co2. The plants are doing very well. Getting pearling on the leafs and fairly good growth. I’m a bit hesatant to dose more than once a week with a fluval fertilizer. I’ve conquered one bout of BBA. Thanks for getting back.